You are browsing content specific to your location, some treatments may not be available:
You are browsing content specific to your location, some treatments may not be available:
Anti-Müllerian hormone (AMH) is an important ovarian reserve marker for baseline assessment and therapeutic strategy in fertility treatments, which is considered reliable when measured on any day of the cycle. Recent data have pointed toward significant fluctuations of AMH and questioned whether a single measurement is reliable for clinical decision-making. The aim of this study was to evaluate whether the AMH does have significant variations during a natural cycle when a fully automated assay is used for the sample analysis. We performed a prospective study including healthy volunteers with regular cycles, from April to December 2017. Blood samples for AMH, FSH, LH, estradiol, and progesterone were obtained on day 2/3, day 10, day of LH surge, luteal phase and day 2/3 of subsequent menses. AMH analysis was performed with Elecsys® AMH automated assay. Trial was registered with clinical.trials.gov: NCT03106272. One hundred samples from 22 women with a mean age of 30.74 ± 0.11 years and a BMI of 23.23 ± 0.63 kg/m2 were analyzed. There was a substantial longitudinal fluctuation in AMH levels, indicated by the coefficient of variation (CV) intra-cycle of 0.2070 ± 0.143. A positive correlation between LH and AMH concentrations was found at the moment of LH rise (p < 0.0001). Absolute intra-individual inter-cyclic variability was 0.75 ng/mL (range: 0.03–2.81 ng/mL) and inter-cycle CV was 0.28 (Confidence interval: 0.16–0.39; p < 0.0001). According to our results, with the use of a fully automated assay in natural cycle, AMH shows significant intra- and inter-cycle variations, which are not caused by analytical variability. Future investigations, evaluating AMH dynamics and the best time for AMH assessment should be conducted.
Introduction
Anti-Müllerian hormone (AMH) is a dimeric glycoprotein produced by granulosa cells of pre-antral and small antral follicles in the ovary and its production is independent of follicle stimulating hormone (FSH) (1). The release of AMH from the granulosa cells results in measurable serum levels and these concentrations have been shown to be proportional to the number of developing follicles in the ovaries (2). Therefore, AMH has emerged as one of the most important clinical indirect markers for ovarian reserve (3) and is used by clinicians for predicting ovarian response to hyperstimulation for IVF, facilitating counseling of patients and individualization of stimulation regimens. Furthermore, a recent publication demonstrated a strong positive, age-independent relationship between AMH level and number of euploid blastocysts obtained following IVF/ICSI cycles (4), also with miscarriage rates (5). It is well recognized that serum AMH displays a high inter-individual variability mainly attributed to the different number in antral follicles within groups of women of similar age (2). It also appears to vary with factors such as hormonal contraceptive use, pregnancy, body mass index, smoking (6), and the use of gonadotropins for ovarian stimulation (7, 8). Serum AMH is generally believed to be a reliable test that may be measured on any day of a natural cycle, with minimal intra-individual variability (6, 9–13). However, recent publications have raised doubts as to the reliability of a single AMH test taken ad-hoc in a natural cycle. Gorkem et al. demonstrated that serum AMH levels seem to be higher during the follicular phase as compared to the luteal phase in infertile women with normal, high, and low ovarian reserve (14). Other authors have described important variability in AMH concentrations during the menstrual cycle, which was deemed higher than the fluctuations expected due to the analysis alone (15, 16). Moreover, it has been proposed that young women may have a pattern of intra-cycle fluctuation that differs from that of older women (17). Circadian fluctuations for AMH serum levels have also been identified (18). These fluctuations in AMH during the natural cycle were previously attributed to analytical variations caused by different conditions used for sample storage and/or the assay method (19). But now, AMH can be analyzed with fully automated AMH assays which are highly sensitive and precise, with a broad linear range (20) allowing for more efficient sample processing and reducing possible procedural errors (20–22). Nowadays, it is widespread accepted that a single AMH measurement as an accurate reflection of a patients’ ovarian reserve and clinicians take this result into serious consideration when counseling women about their reproductive health. In light of recent publications demonstrating AMH variability both throughout the menstrual cycle and between consecutive cycles, questions rise as to whether a single AMH measurement would be reliable. The aim of this study was to investigate AMH serum levels in healthy, cycling women using fully automated assay ElecsysⓇ AMH (Roche, for Cobas 601 platformⓇ), at defined time-points of the natural cycle and short-term inter-cycle variations. Materials and Methods Subjects Twenty-two healthy volunteers were included in this study, which was undertaken from April to December 2017, in IVIRMA Abu Dhabi clinic. Women between the ages of ≥18 to ≤ 38 years old, with regular menstrual cycles between 28 and 32 days and BMI between ≥18 and ≤ 28 kg/m2 were included. Exclusion criteria included the following factors: the intake of hormonal contraceptives for a minimum of 2 months immediately prior to study commencement, pregnancy, breastfeeding, and previous conditions which may adversely affect ovarian reserve (ovarian surgery, chemotherapy, pelvic radiation). No volunteers with infertility background were included. The participants fulfilled a written questionnaire including menstrual cycle pattern, previous pregnancies, deliveries and miscarriages, ethnicity, smoking and possible exclusion criteria. They were informed about the methods, sample processing and objectives of the study and all of them signed informed consent. Blood Sampling Blood samples were obtained by venepuncture in the clinic between 8 am and 2 pm, transferring the samples immediately to the laboratory located in the same center for immediate processing and further analysis. During the natural cycle, samples were collected on day 2/3 (AMH_01), day 10 (mid follicular phase, AMH_MFP), day of LH rise (AMH_LHR), mid luteal phase (AMH_MLP), and day 2/3 of the subsequent menstruation (AMH_02). The LH surge was defined for the purpose of our study to have begun when the concentration of LH rose by 180% above the latest serum value available in that patient and continued to rise thereafter (23). For this purpose, LH, estradiol and progesterone were monitored starting on day 10 of a natural cycle and then every 3 days until LH surge was diagnosed (24, 25). The luteal phase was confirmed by an elevated serum progesterone level (>3 ng/ml) 8 days after LH surge.
One sample of 5 mL of blood was collected each time. Blood was centrifuged and serum separated in two parts: one for AMH analysis and one for gonadotropin and steroid hormone analysis. Analysis of FSH, LH, estradiol and progesterone was performed using a competitive immunoassay manufactured by Roche on the Cobas 601 platform®. Results for these hormones were obtained and evaluated on the same day of blood collection. Serum samples for AMH were aliquoted, frozen at −20°C the same day of collection and stored for batch analysis. All samples from each study participant were analyzed under the same conditions on the same day, using Elecsys® AMH automated assay (for Cobas 601 platform, Roche®) according to manufacturer‘s instructions. Imprecision expected from the assay was <5%, as described by the manufacturer; intra-assay and inter-assay co-efficient of variation for Elecsys® AMH automated assay has been reported as 0.5–1.4 and 0.7–1.9%, respectively (26).
Statistical Analysis
A research database was generated for all the variables that were evaluated. The export data were correctly anonymized and analyzed with SPSS 22.0.Demographic variables and hormonal values were summarized with mean and standard deviation. In order to describe hormones and their dynamics, we determined the mean value and standard deviations each time and represented it with boxplot figures. Moreover, we performed polynomial interpolation for dynamic values and represented the variations with mean polynomial interpolation and variance band.
AMH values were represented for AMH dynamic description using a 4-degree-polynomial-exponential interpolation of data. This polynomial-exponential interpolation was used as well to impute missing data (10 missing data points). Patients were not categorized according to initial AMH since homogeneous variability in cluster groups could not be previously assumed.
Pearson correlation test was performed between AMH values and other hormones along with temporary marks and clustering analyses were done for correlation between AMH dynamic patterns and demographics parameters. For the evaluation of any AMH dynamic patterns related with demographics parameters, we applied clustering analyses over AMH polynomial coefficients that were previously calculated. A p-value of 0.05 was considered statistically significant.
Ethical Approval
The study was approved by the internal Ethics Committee under the number REFA011 and registered for clinical.trials.gov: NCT03106272.
Results
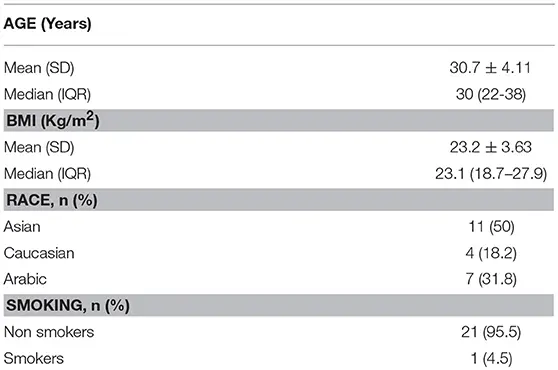
A total of 100 samples from the 22 women were analyzed, with a mean age of 30.7 ± 4.11 years and a BMI of 23.2 ± 3.63 Kg/m2 (Table 1). All the samples were analyzed with Elecsys® AMH automated assay for Cobas 601 platform (Roche®).
To read full publication download below